Device—Breakthrough Device Designation
This section introduces the FDA Breakthrough Device Designation (BDD), a designation aimed at accelerating the review and approval process for medical devices that offer significant benefits over existing treatments. By offering priority FDA review, BDD enables faster regulatory approval and market access for groundbreaking technologies.
MDCE provides expert guidance throughout the BDD application process, helping clients navigate complex requirements, ensure compliance, and bring life-saving innovations to market efficiently.
Introduction of FDA Breakthrough Device Program
- What is FDA Breakthrough Device Designation?
The FDA Breakthrough Device Designation (BDD) accelerates the review and approval of medical devices that provide significant advantages over existing treatments. It focuses on devices addressing critical unmet needs in life-threatening or debilitating conditions, ensuring faster access to transformative solutions for patients.
Devices with BDD receive priority FDA review, expediting regulatory pathways such as premarket approval (PMA), 510(k) clearance, and De Novo classification. This designation reduces time-to-market for devices with the potential to dramatically improve patient outcomes.
- Challenges in Breakthrough Device Designation
Securing Breakthrough Device Designation (BDD) involves meeting stringent criteria and navigating complex regulatory pathways. Key challenges include demonstrating significant advantages over existing alternatives, such as reducing hospitalization, improving patient quality of life, enabling self-directed care, or establishing long-term clinical efficiencies. Additionally, preparing comprehensive evidence to support these claims is critical. Successfully addressing these demands requires strategic planning, deep regulatory expertise, and effective communication with the FDA.
- Key Benefits of Breakthrough Device Designation
The Breakthrough Device Designation expedites FDA review, significantly reducing the time-to-market for medical devices addressing serious health conditions. This fast-track approach allows for quicker patient access to innovative treatments.
Additionally, the designation aids in streamlining entry into international markets, enhancing the global reach of critical medical technologies.
Core Services From MDCE in BDD

Comprehensive Support for BDD Applications
MDCE provides end-to-end assistance throughout the Breakthrough Device Designation (BDD) application process. From initial submission preparation to direct communication with the FDA, we guide clients through every critical step. Our team ensures all application elements are meticulously developed to meet regulatory expectations, enabling a smoother path toward designation.
Tailored Regulatory Strategies
Understanding the unique features of each medical device, MDCE crafts customized regulatory strategies designed to align with both FDA requirements and the device’s specific characteristics. This tailored approach ensures that the strengths of each device are highlighted, significantly improving the chances of approval under the BDD program.
Comprehensive Documentation Preparation
MDCE assists in compiling all required documentation to meet stringent FDA standards. This includes preparing detailed device descriptions, performance summaries, standards of care comparisons, and supporting clinical evidence. By ensuring accuracy and completeness in every submission, we streamline the process and strengthen the application’s impact.
Primary Advantages of MDCE
With a strong foundation of leadership excellence and extensive industry experience, MDCE offers unparalleled regulatory support to medical device companies navigating complex global approval processes. Anchored by a team of seasoned experts, MDCE ensures seamless regulatory submissions, strategic compliance, and efficient navigation through stringent FDA and international standards.
Substantial Regulatory Expertise
MDCE brings deep expertise in FDA and global regulations, ensuring seamless submissions and strict compliance. Our team stays updated on regulatory changes, providing clients with accurate and timely guidance for their applications.
Strategic Relationships with Key Stakeholders
With strong relationships across the FDA, clinical experts, and global institutions, MDCE navigates complex regulatory processes effectively. These connections enable us to offer clients unparalleled support in achieving market approval for their devices.
Proven Track Record in Breakthrough Device Designation
MDCE has a proven track record of securing Breakthrough Device Designation for numerous clients. This success demonstrates our ability to handle regulatory challenges and builds trust with new clients seeking approval for innovative medical devices.
Case Studies and Project Experience
With extensive expertise in navigating complex regulatory landscapes, MDCE has a proven track record of successfully securing Breakthrough Device Designation (BDD) for innovative medical devices across multiple therapeutic areas. Our comprehensive services and strategic insights enable clients to achieve faster regulatory approvals and bring life-changing technologies to global markets

Innovative Magnesium Alloy Hollow Screw Secures FDA Breakthrough Device Designation
In January 2025, MDCE successfully supported a global MedTech innovator in securing FDA Breakthrough Device Designation (BDD) for its revolutionary magnesium alloy hollow screw, marking a pivotal milestone amid the FDA's increasingly rigorous review landscape. This achievement underscores the device's transformative design and profound clinical value, while further solidifying MDCE's leadership in delivering world-class regulatory strategies and expediting global market entry for next-generation medical technologies.
MDCE’s Contributions:
-
Regulatory Strategy: MDCE developed a tailored submission strategy, aligning with FDA requirements and providing robust evidence to demonstrate the product's superiority over existing treatments.
-
Global Expertise: Leveraging its deep understanding of FDA regulations, MDCE streamlined the approval process, ensuring compliance and addressing regulatory challenges efficiently.
-
End-to-End Support: MDCE provided comprehensive services, including technical documentation preparation, regulatory communication, and compliance assurance, enabling a smooth and successful certification process.
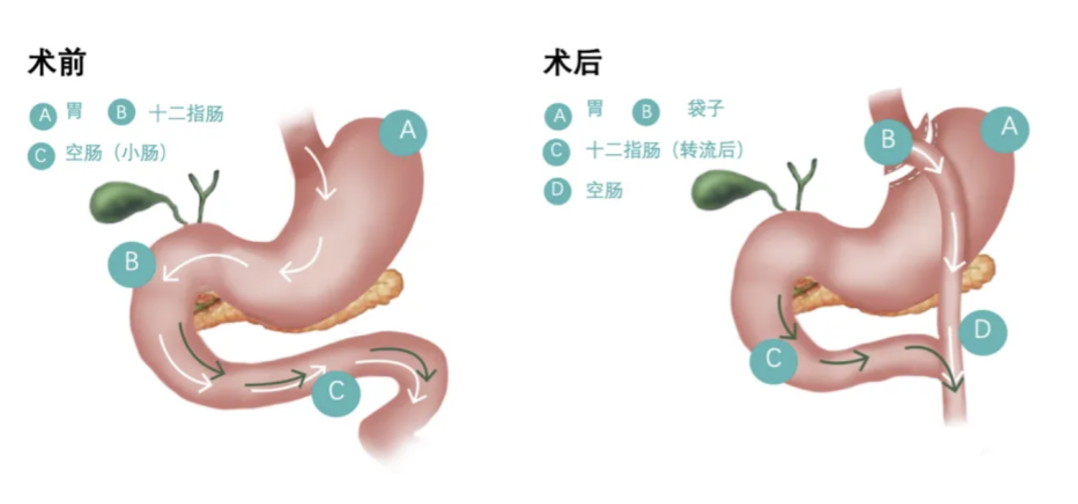
Innovative Gastric Bypass Stent Secures FDA Breakthrough Device Designation
In October 2024, MDCE successfully supported a pioneering MedTech company in achieving FDA Breakthrough Device Designation (BDD) for its innovative gastric bypass stent system. This milestone not only highlights the system's potential to transform the treatment of metabolic diseases worldwide but also reinforces the growing impact of cutting-edge medical technologies in addressing global healthcare challenges.
Leveraging its global academic network and regulatory expertise, MDCE provided comprehensive support to fast-track this breakthrough device, setting the stage for international market success
MDCE’s Contributions:
- Regulatory Strategy: MDCE restructured the submission approach, aligning it with FDA requirements and providing robust evidence to support the designation.
- Global Expertise: The team addressed compliance challenges and streamlined the regulatory process, enabling a faster approval timeline.
- End-to-End Support: MDCE delivered tailored services, including preparing technical documentation, navigating regulatory inquiries, and ensuring full compliance with FDA standards.
Contact Us
Ready to Fast-Track Your Breakthrough Device?
Get in touch with MDCE to begin the FDA Breakthrough Device Designation process. Our experts will guide you through every step, ensuring a smooth path to accelerated approval and market access.
Contact Information:
- Phone: 0512-6280 1222
- Email: ask@mdcecro.com
- Address: Room 2003-1, Building 2, Huihu Building, No. 10 Moon Bay Road, Suzhou Industrial Park, Suzhou City
MDCE’s expertise in Breakthrough Device Designation ensures that your innovative medical device gets the attention it deserves. We fast-track your device to market, helping save lives and providing quicker access to cutting-edge treatments. Let MDCE help you navigate the complex regulatory landscape and bring your breakthrough innovation to market faster.
.png?width=188&height=60&name=LOGO-%E5%8E%9F%E8%89%B2%E9%95%BF%E6%A0%87%20(1).png)
